Abstract
Introduction: Induction therapy consisting of dasatinib (DAS) and corticosteroids (CS) yields high rates of morphologic complete response (CR) in adults w/ newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). However, only ~20% of patients (pts) achieve BCR-ABL1 response depth of <10-3 at day (d) 22 or complete molecular response (CMR) at d85, which are associated w/ greater relapse-free survival (Foa et al., Blood, 2011). In healthy C57BL/6 mice bearing BCR-ABL+ Arf-/- ALL, DAS restores cytokine dependency and sensitizes leukemic cells to ruxolitinib (RUX). RUX may enhance response to DAS by abrogating cytokine signaling, suggesting a potential benefit to combination targeted therapy in the frontline setting (Applemann et al., Blood, 2015).
Methods: We conducted a phase I trial (NCT02494882) w/ accelerated dose escalation of RUX. Pts w/ newly diagnosed Ph+ ALL received CS pre-phase (d -6 to 0) followed by DAS 140 mg daily (starting d1) in combination w/ dexamethasone (DEX) 10 mg/m2 PO daily (d1-24, then taper) and RUX (d1-84). RUX doses were 10 mg PO BID (n=1), 15 mg PO BID (n=1), 20 mg PO BID (n=1), and 25 mg PO BID (n=7). IT methotrexate was given as CNS prophylaxis (d22, 43, 64, 85). Post-induction therapy was left to the discretion of the treating physician. The primary objective was to determine the recommended phase 2 dose (RP2D) of RUX in this combination. Secondary objectives included assessment of response kinetics, including minimal residual disease (MRD) negativity by flow cytometry (FACS) and BCR-ABL1 transcript monitoring, and ABL kinase mutation development.
Results: Ten pts enrolled (6M, 4F). Median age at time of screening bone marrow (BM) studies was 63.8 yrs (range, 25.7-77.4; 7/10 age ≥60). BCR-ABL1 transcripts were p190 (n=7), p210 (n=2), atypical (n=1). No dose-limiting toxicity (DLT) has been observed. Enrollment was expanded at the highest RUX dose level (25 mg PO BID). Grade (Gr) 3-4 non-hematologic toxicities possibly/probably attributable to DAS/RUX/CS were limited to Gr 3 hyperglycemia in the setting of CS (n=5), Gr 3 hypofibrinogenemia (n=1), and Gr 3 peripheral edema (n=1). Two pts discontinued DAS after completing the 84d induction due to symptomatic pleural effusions.
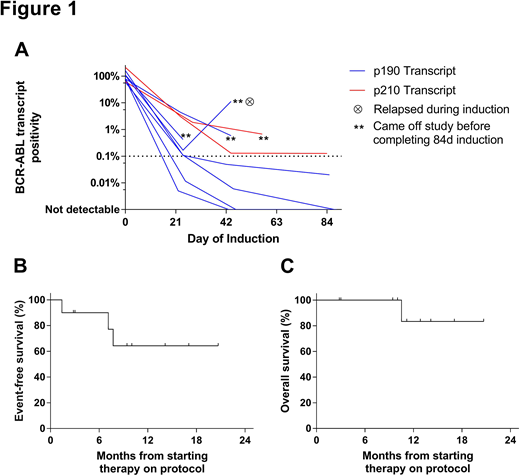
All pts achieved morphologic CR or CR w/ incomplete hematologic recovery (CRi). Median log reduction in BCR-ABL1 transcript levels (Figure 1A) was 2.7 at d22 (range, 1.3-4.2, n=9), 3.4 at d43 (range, 1.0-CMR, n=8), and CMR at d85 (range, 3.2-CMR, n=5). By FACS, 7/10 achieved MRD <0.01% (undetectable in 3/10). By PCR, 3/10 achieved CMR. Six pts remained on study through d85. Of these pts, 6/6 achieved MRD <0.01% by FACS (undetectable in 3/6); 3/5 w/ previously quantifiable BCR-ABL1 transcript levels achieved CMR. Four pts were withdrawn from study before d85 for morphologic relapse at d43 w/ new T315I ABL kinase mutation (n=1), new T315I mutation w/o relapse (n=1), suboptimal response kinetics despite achieving CR (n=1), and complications related to a splenic abscess in CR (n=1).
Among 6 pts completing the 84d induction, next post-remission therapy included tyrosine kinase inhibitor (TKI) + concurrent blinatumomab (n=3), TKI + hyperCVAD (n=1), and allogeneic hematopoietic cell transplantation (alloHCT, n=1). Of the 4 pts not completing the 84d induction, next therapy included ponatinib + blinatumomab (n=2), ponatinib + hyperCVAD (n=1), or DAS alone (n=1). Overall, 3 pts experienced morphologic relapse, which was observed in the setting of T315I (n=2) or F317L (n=1) ABL kinase mutations. Median EFS and OS (Figure 1B&C) are not yet reached in this small group of pts at median follow-up of 11.2 months among survivors (range, 2.8-20.7).
Conclusions: Chemotherapy sparing-induction consisting of DAS, RUX, and initial DEX was well tolerated among adults w/ newly-diagnosed Ph+ ALL and led to CR/CRi in all pts. The RP2D of RUX is 25 mg PO BID. Kinetics of BCR-ABL1 transcript reduction compare favorably w/ other series of TKI + steroid-based induction, w/ rapid achievement of median >3 log reduction in BCR-ABL1 transcripts. Correlation of response kinetics w/ JAK/STAT pathway inhibition may suggest mechanisms of resistance apart from ABL kinase mutations. In future chemotherapy-sparing induction approaches, incorporating ponatinib, asciminib, or other novel therapies (vs. DAS) during induction may suppress leukemic clones bearing T315I mutations and reduce early relapse rates.
Geyer:Dava Oncology: Honoraria. King:Genentech: Other: Advisory Board . Mauro:Bristol-Myers Squibb: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Takeda: Consultancy. Tallman:BioSight: Other: Advisory board; AROG: Research Funding; AbbVie: Research Funding; Cellerant: Research Funding; ADC Therapeutics: Research Funding; Daiichi-Sankyo: Other: Advisory board; Orsenix: Other: Advisory board. Levine:Gilead: Honoraria; Isoplexis: Equity Ownership; Epizyme: Patents & Royalties; Roche: Consultancy, Research Funding; Loxo: Consultancy, Equity Ownership; Novartis: Consultancy; C4 Therapeutics: Equity Ownership; Qiagen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Prelude: Research Funding; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Research Funding; Imago: Equity Ownership. Douer:Jazz Pharmaceuticals: Consultancy; Gilead Sciences: Consultancy; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria; Spectrum: Consultancy. Park:Novartis: Consultancy; Kite Pharma: Consultancy; Adaptive Biotechnologies: Consultancy; AstraZeneca: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Shire: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal